Helping Sonographers
Nuchal Translucency Ultrasound and First Trimester Screening |
|
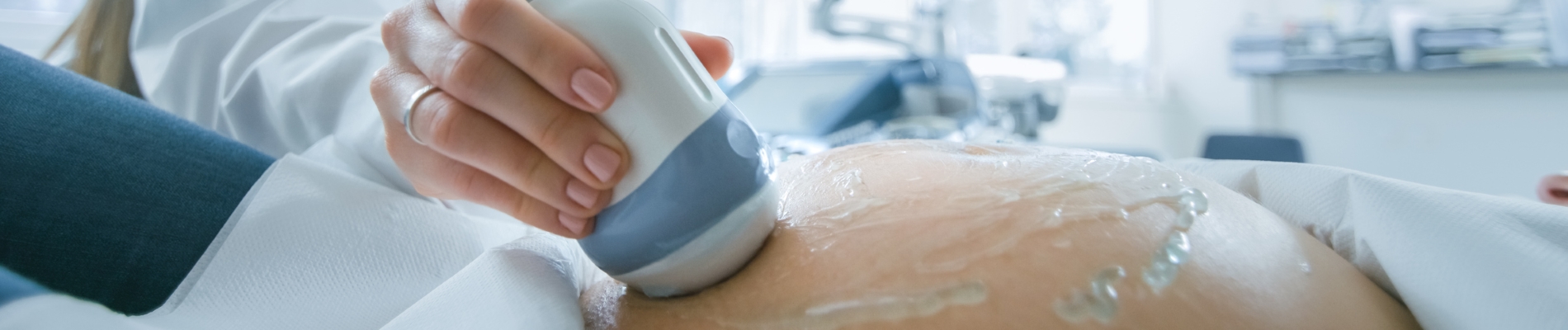
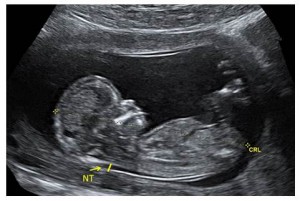
The fetal nuchal translucency (NT) is a collection of fluid that is present at the back of the fetal neck in the first trimester of pregnancy. Increased NT thickness can be associated with chromosomal abnormalities, cardiac defects, and/or other genetic syndromes. A NT certified sonographer performs an ultrasound on a pregnant individual to measure the thickness of the fetal NT when the fetal crown rump length (CRL) is between 45mm and 84mm (approximately 11 ̶ 14 weeks gestation). If the pregnant individual has requested first trimester screening, this NT measurement is submitted to one of the three Ontario Multiple Marker Screening (MMS) laboratories along with a blood sample. The Ontario labs use this measurement along with biographical information and biochemical markers from the maternal serum to calculate individual risk for a pregnancy affected with a chromosomal abnormality (e.g., Down syndrome, Trisomy 18).
|
BORN Ontario Data |
|
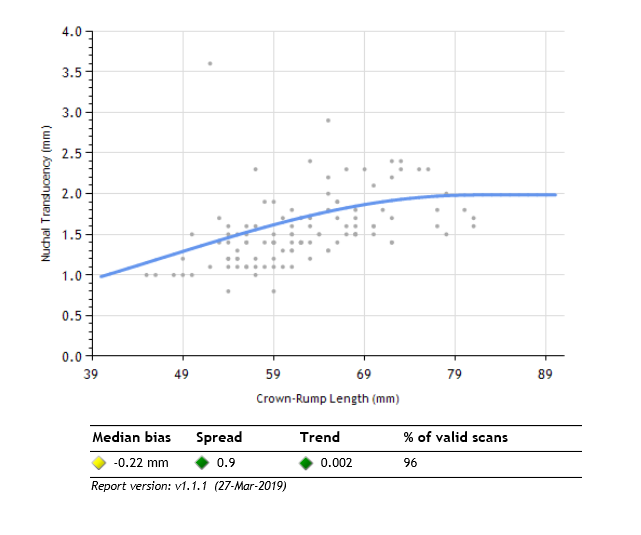
Prenatal Screening Ontario (PSO), a program housed within BORN Ontario, is using BORN Ontario data to develop a nuchal translucency quality assurance (NTQA) program to support sonographers in taking high quality NT measurements. Since 2012, BORN Ontario has collected NT ultrasound measurement data as recorded on pregnant individuals' first trimester screening requisitions. They have correlated this data with individual NT ID numbers belonging to certified sonographers and have created personalized NT performance distributions for each scanner. Personalized performance distributions reflect sonographers' NT measurement performance when compared against the expected normal population distribution established by the Fetal Medicine Foundation UK (an ultrasound governing body). Sonographers can gain access to their personalized NT performance distributions via the BORN Information system (BIS). These performance distributions give sonographers insight into their scanning habits and they use this information to inform their practice and improve their scanning performance. |
Prenatal Screening Ontario's Nuchal Translucency Quality Assurance Program |
|
Both international and national professional bodies recommend that sonographers participate in a formal NTQA program if taking NT measurements. Without participation in such a program, measurement quality and first trimester screening quality deteriorates over time. Guided by the aforementioned BORN Ontario data and a panel of experts in diagnostic imaging and prenatal screening, PSO is developing a NTQA program for Ontario that is:
There are two vital tools used to assess NT measurement performance:
As PSO implements the NTQA program, they are working to support sonographers in their practise by using these tools to assess their NT measurement performance and provide them with actionable feedback to help them improve and/or maintain their skills. Visit the PSO website for more information on NT certification and Ontario's NTQA programSonographers and physicians who perform, or who would like to perform NT measurements and submit them to the Ontario MMS labs for prenatal screening must obtain an Ontario NT ID number from the Ontario MMS labs and register with PSO at BORN Ontario. For more information on the following, please visit the PSO website.
|


 Subscribe to this page
Subscribe to this page
